
24
PLoS One. 2012;7(12):e53242.
Resident CD11b(+)Ly6C(-) lung dendritic cells are
responsible for allergic airway sensitization to house
dust mite in mice.
Mesnil C, Sabatel CM, Marichal T, Toussaint M, Cataldo D, Drion PV, Lekeux P, Bureau F,
Desmet CJ.
Abstract
Conventional dendritic cells (DCs) are considered to be the prime initiators of airway allergy.
Yet, it remains unclear whether specifc DC subsets are preferentially involved in allergic
airway sensitization. Here, we systematically assessed the respective pro-allergic potential
of individually sorted lung DC subsets isolated from house dust mite antigen (HDM)-treated
donor mice, following transfer to naïve recipients. Transfer of lung CD11c(+)CD11b(+) DCs,
but not CD11c(+)CD11b(-)CD103(+) DCs, was sufcient to prime airway allergy. The CD11c(+)
CD11b(+) DC subpopulation was composed of CD11c(+)CD11b(+)Ly6C(+) infammatory
monocyte-derived cells, whose numbers increase in the lungs following HDM exposure, and
of CD11c(+)CD11b(+)Ly6C(-) DCs, which remain stable. Counterintuitively, only CD11c(+)
CD11b(+)Ly6C(-) DCs, and not CD11c(+)CD11b(+)Ly6C(+) DCs, were able to convey antigen
to the lymph nodes and induce adaptive T cell responses and subsequent airway allergy. Our
results thus support that lung resident non-infammatory CD11c(+)CD11b(+)Ly6C(-) DCs are
the essential inducers of allergic airway sensitization to the common aeroallergen HDM in
mice.
J Immunol. 2012 Oct 15;189(8):4135-43.
Control of allergen-induced infammation and
hyperresponsiveness by the metalloproteinase
ADAMTS-12.
Paulissen G, El Hour M, Rocks N, Guéders MM, Bureau F, Foidart JM, Lopez-Otin C, Noel A,
Cataldo DD.
Abstract
A disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) constitute a
family of endopeptidases related to matrix metalloproteinases. These proteinases have been
largely implicated in tissue remodeling associated with pathological processes. Among them,
ADAMTS12was identifed as an asthma-associated gene in a human genome screening program.
However, its functional implication in asthma is not yet documented. The present study aims
at investigating potential ADAMTS-12 functions in experimental models of allergic airways
disease. Two diferent
in vivo
protocols of allergen-induced airways disease were applied
to the recently generated Adamts12-defcient mice and corresponding wild-type mice. In
this study, we provide evidence for a protective efect of ADAMTS-12 against bronchial
infammation and hyperresponsiveness. In the absence of Adamts12, challenge with diferent
allergens (OVA and house dust mite) led to exacerbated eosinophilic infammation in the bron-
choalveolar lavage fuid and in lung tissue, along with airway dysfunction assessed by increased
airway responsiveness following methacholine exposure. Furthermore, mast cell counts and
ST2 receptor and IL-33 levels were higher in the lungs of allergen-challenged Adamts12-
defcient mice. The present study provides, to our knowledge, the frst experimental evi-
dence for a contribution of ADAMTS-12 as a key mediator in airways disease, interfering with
immunological processes leading to infammation and airway hyperresponsiveness.
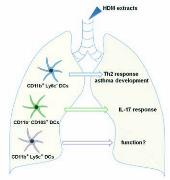
Resident non-infammatory CD11b+Ly6C-
dendritic cells are the prime inducers of
airway allergic sensitization.
The results of this study support a model in which
only CD11b+Ly6C- lung dendritic cells (DCs) are
able to prime Th2 adaptive responses and hence
to sensitize mice to airway allergy to house dust
mites. CD11b-CD103+ DCs prime IL-17 pro-
duction in the lung draining lymph nodes. In spite
of their signifcant allergen uptake in the lung,
CD11b+Ly6C+ DCs are devoid of Th2 priming
activity and their function remains to be deter-
mined.